AAV Gene Therapy Market Size to Skyrocket at 40.1% CAGR by 2034
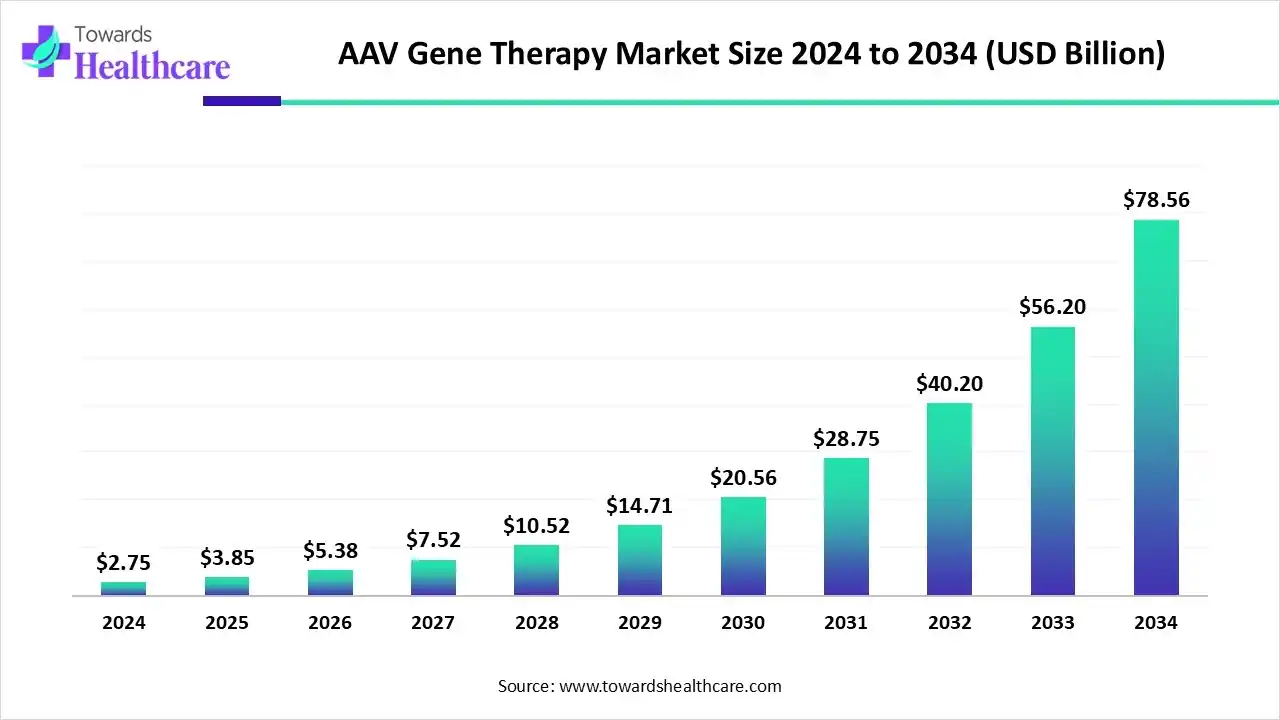
The AAV gene therapy market size is calculated at USD 3.85 billion in 2025 and is expected to reach around USD 78.56 billion by 2034, growing at a CAGR of 40.1% for the forecasted period.
Ottawa, Oct. 07, 2025 (GLOBE NEWSWIRE) -- The global AAV gene therapy market size was valued at USD 2.75 billion in 2024 and is predicted to hit around USD 78.56 billion by 2034, rising at a 40.1% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
The growth of the market is driven by the growing research and development investments, advancements in technologies to increase efficiency, and the rising prevalence of genetic disorders, which are factors that drive the growth of the market.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5810
Key Takeaways
- North America held a major revenue share of 54% of the market in 2024.
- Asia-Pacific is expected to grow at the fastest CAGR of 27–29% in the market during the forecast period.
- By therapeutic area, the neurological disorders segment dominated the global market with a revenue share of 39% in 2024.
- By therapeutic area, the hematologic disorders segment is expected to witness the fastest growth with a 32% CAGR in the AAV gene therapy market over the forecast period.
- By vector serotype, the AAV9 segment contributed the biggest revenue share of 43% of the market in 2024.
- By vector serotype, the engineered/hybrid capsids segment is expected to expand rapidly with a 36% CAGR in the market in the coming years.
- By route of administration, the intravenous segment registered its dominance over the global market with 58% revenue in 2024.
- By route of administration, the intrathecal segment is expected to grow at the fastest CAGR of 26% CAGR during the forecast period.
- By application stage, the commercialized therapies segment led the global market with 35% revenue in 2024.
- By application stage, the preclinical therapies segment is expected to grow at the fastest CAGR of 28% in the market during the forecast period.
- By manufacturing type, the in-house manufacturing segment held the largest revenue share of 60% of the AAV gene therapy market in 2024.
- By manufacturing type, the contract development and manufacturing organizations (CDMOs) segment is expected to show the fastest growth with a 30% CAGR in the upcoming years.
- By end-user, the pharmaceutical and biotech companies segment held a dominant presence in the market with 66% in 2024.
- By end-user, the CDMOs/vector production facilities segment is expected to grow with the highest CAGR of 23% in the market during the studied years.
Market Overview & Potential
The AAV gene therapy market refers to the market for therapies based on adeno-associated virus (AAV) vectors, a leading delivery platform for in vivo gene therapy. AAV vectors are non-pathogenic and have low immunogenicity, making them ideal for long-term gene expression in non-dividing and slowly dividing cells. This market encompasses AAV-based therapeutics, vector manufacturing technologies, clinical-stage therapies, and commercial products used to treat rare genetic disorders, neurological diseases, ophthalmic conditions, hematologic disorders, and other conditions.
Market Scope
| Table | Scope | |
| Market Size in 2025 | USD 3.85 Billion | |
| Projected Market Size in 2034 | USD 78.56 Billion | |
| CAGR (2025 - 2034) | 40.1 | % |
| Leading Region | North America by 54% | |
| Market Segmentation | By Therapeutic Area, By Vector Serotype, By Route of Administration, By Application Stage, By Manufacturing Type, By End User, By Region | |
| Top Key Players | Novartis (AveXis), Spark Therapeutics (Roche), BioMarin Pharmaceutica, Sarepta Therapeutics, uniQure, Regenxbio, Audentes Therapeutics (Astellas), Voyager Therapeutics, MeiraGTx, Asklepios BioPharmaceutical (AskBio - Bayer), Krystal Biotech, Genethon, BridgeBio Gene Therapy, Passage Bio, Solid Biosciences, Freeline Therapeutics, Capsida Biotherapeutic, LogicBio Therapeutics, Taysha Gene Therapies, Iveric Bio (part of Astellas) | |
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What is the Growth Potential Responsible for The Growth of The AAV Gene Therapy Market?
The main factors driving the AAV gene therapy market are the rising rates of genetic disorders such as hemophilia and muscular dystrophy, major progress in gene editing technologies, a favorable regulatory climate with accelerated approvals, and strong R&D investments from both public and private sectors. The market is also expanding beyond rare diseases into fields like cardiovascular and metabolic conditions, supported by improving healthcare infrastructure in emerging economies. Strategic partnerships between companies and research institutions, along with the increasing success of clinical trials, further boost market growth.
What Are the Growing Trends Associated with the AAV Gene Therapy Market?
Increased Clinical Pipeline:
- There are over 2,000 gene therapies in clinical development, with AAV vector-based therapies seeing a 25% increase in clinical trial activity in recent years.
Expansion to New Applications:
- New therapeutic areas, such as cardiovascular and metabolic diseases, are emerging as potential applications for AAV-based therapies.
Focus on Manufacturing & Cost-Effectiveness:
- Strategic investments in scalable and cost-effective AAV vector production are crucial to meeting market demand.
Growth in Contract Development and Manufacturing (CDMOs):
- The market for CDMOs specializing in AAV vector production is expanding due to maturing clinical pipelines and increased capital deployment.
What Is the Growing Challenge in the AAV Gene Therapy Market?
AAV gene therapy faces challenges, including manufacturing hurdles (scale-up, complexity, high costs), immunogenicity (pre-existing antibodies, immune responses), limited payload capacity for the virus, issues with delivery and targeting efficiency, and long-term safety concerns. Overcoming these barriers requires technological advancement, process standardization, and regulatory efforts to ensure broader accessibility and market adoption.
Regional Analysis
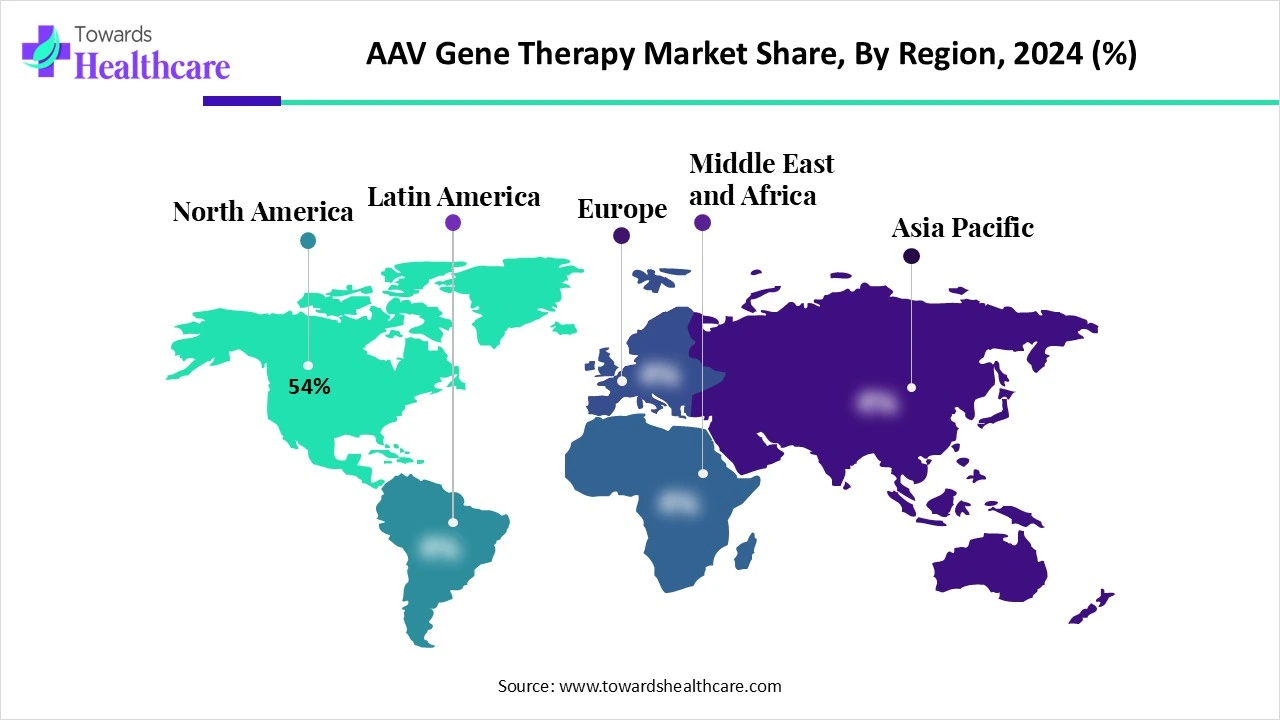
How Did North America Dominate the AAV Gene Therapy Market in 2024?
North America, in 2024, dominated the AAV gene therapy market, driven by a robust biotech ecosystem, advanced healthcare infrastructure, and significant R&D investment. The region benefits from strong support by regulatory bodies like the FDA, which has accelerated approvals of gene therapies through fast-track and orphan drug designations. Leading pharmaceutical companies and academic institutions in the U.S. and Canada continue to pioneer clinical trials and commercialization of AAV-based treatments, particularly for rare genetic disorders and inherited retinal diseases. High healthcare spending and favorable reimbursement models further solidify North America’s position as the global hub for gene therapy innovation.
United States: Leading the Global AAV Gene Therapy Market
The United States is the global leader in the AAV gene therapy market, accounting for a significant share of both clinical trials and commercial approvals. This dominance is driven by a well-established biotech and pharmaceutical ecosystem, extensive funding from both private investors and government initiatives (such as the NIH and Operation Warp Speed), and a streamlined regulatory pathway supported by the FDA’s orphan drug, breakthrough, and fast-track designations. The presence of major players such as Spark Therapeutics, Novartis, and Pfizer, along with leading academic institutions, accelerates innovation and commercialization. High patient awareness, favorable reimbursement policies, and access to cutting-edge healthcare make the U.S. the primary launchpad for new AAV-based therapies targeting rare and monogenic diseases.
What Made the Asia Pacific Significantly Grow in The AAV Gene Therapy Market In 2024?
The Asia-Pacific region is emerging as the fastest-growing market for AAV gene therapy, fueled by increasing government support, rising prevalence of genetic disorders, and growing investment in biotech research. Countries like China, Japan, South Korea, and India are ramping up clinical research capabilities, expanding their regulatory frameworks, and encouraging public-private partnerships to advance gene therapy development. Additionally, the region's large patient population, improving access to diagnostics, and cost-effective manufacturing capacity make it a strategic hotspot for AAV-based therapies. As more domestic biotech firms enter the space, Asia-Pacific is poised to significantly reshape the global gene therapy landscape in the coming years.
China AAV Gene Therapy Market Trends:
China is emerging as the fastest-growing market for AAV gene therapy in the Asia-Pacific region, fueled by government initiatives like the "Healthy China 2030" plan and significant investments in domestic biotech innovation. The country is rapidly expanding its clinical trial landscape, regulatory flexibility, and manufacturing capabilities to support gene therapy development. Chinese firms such as CanSino Biologics, Innovent Biologics, and Viva Biotech are increasingly entering the gene therapy space through partnerships and in-house R&D. Additionally, China's vast patient pool, lower clinical trial costs, and growing talent base make it an attractive market for both local and international companies. As the regulatory framework continues to mature and align more closely with international standards, China is poised to become a major player in global AAV gene therapy development.
Download the single region market report @ https://www.towardshealthcare.com/price/5810
Segmental Insights
By therapeutic area,
The neurological disorders segment dominated the global market with a revenue share of 39% in 2024. AAV-based therapies are widely applied in neurological conditions such as spinal muscular atrophy and Parkinson’s disease. Their ability to cross the blood-brain barrier makes them highly effective for targeting central nervous system disorders. Growing clinical research and regulatory approvals are expanding adoption in this therapeutic domain.
The hematologic disorders segment is expected to witness the fastest growth with a 32% CAGR in the AAV gene therapy market over the forecast period. In hematologic disorders, AAV vectors deliver corrective genes for conditions like hemophilia. Their long-term gene expression and safety profile make them suitable for blood-related diseases. Increasing clinical trial success and patient demand for curative therapies are driving momentum in this segment, with strong commercial opportunities emerging.
By vector serotype,
The AAV9 segment contributed the biggest revenue share of 43% of the market in 2024. AAV9 is one of the most widely used serotypes due to its natural ability to target muscle and neural tissues. It underpins several approved therapies and continues to dominate clinical pipelines. Its proven efficacy and safety record make it a cornerstone in advancing genetic medicine applications.
The engineered/hybrid capsids segment is expected to expand rapidly with a 36% CAGR in the market in the coming years. Engineered and hybrid capsids are developed to overcome limitations of natural serotypes, including immune response and limited tissue tropism. These customized vectors enable improved targeting, reduced dosing, and enhanced therapeutic efficiency. With ongoing innovation, this segment represents the future of precision AAV-based therapy development across multiple indications.
By route of administration,
The intravenous segment registered its dominance over the global market with 58% revenue in 2024. Intravenous delivery is the most common route for systemic AAV therapies, enabling widespread gene distribution. It is widely used in neuromuscular and metabolic disorders. However, immune response challenges persist, prompting researchers to refine dosage strategies and vector designs to enhance safety while maintaining therapeutic efficacy.
The intrathecal segment is expected to grow at the fastest CAGR of 26% CAGR during the forecast period. Intrathecal administration provides direct delivery into cerebrospinal fluid, enhancing central nervous system targeting while minimizing systemic exposure. It is increasingly applied in rare neurological disorders. This method improves therapeutic concentration in the brain and spinal cord, making it a growing focus for advanced AAV gene therapy programs.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
By the application stage,
The commercialized therapies segment led the global market with 35% revenue in 2024. Commercialized AAV therapies, such as those for spinal muscular atrophy and retinal disorders, demonstrate the market’s clinical success. With strong regulatory approvals, these therapies are setting new benchmarks in patient outcomes. Their commercial performance validates AAV’s transformative potential and encourages further pipeline expansion.
The preclinical therapies segment is expected to grow at the fastest CAGR of 28% in the market during the forecast period. Preclinical therapies represent the majority of AAV-based programs, targeting diverse indications from oncology to rare metabolic conditions. Significant investment and innovation in this stage highlight the growing confidence in AAV platforms. Success in preclinical development ensures a steady pipeline of future commercial therapies.
By manufacturing type,
The in-house manufacturing segment held the largest revenue share of 60% of the AAV gene therapy market in 2024. Pharmaceutical and biotech companies with in-house manufacturing capabilities retain control over production, quality, and intellectual property. This model supports rapid scaling and innovation but requires significant capital investment. Larger firms often choose this route to secure supply chains for their gene therapy portfolios.
The contract development and manufacturing organizations (CDMOs) segment is expected to show the fastest growth with a 30% CAGR in the upcoming years. CDMOs provide critical expertise, infrastructure, and scalability for companies lacking in-house capabilities. They are pivotal in accelerating clinical-to-commercial transitions while reducing operational costs. Rising outsourcing trends are making CDMOs essential partners, particularly for smaller biotech firms advancing AAV gene therapy programs globally.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
By end-user,
The pharmaceutical and biotech companies segment held a dominant presence in the market with 66% in 2024. Pharmaceutical and biotech companies drive innovation in AAV gene therapy, from discovery to commercialization. They lead R&D investments and establish collaborations with academic institutes and CDMOs. Their role in expanding therapeutic pipelines and ensuring market growth remains central to the adoption of AAV-based treatments.
The CDMOs/vector production facilities segment is expected to grow with the highest CAGR of 23% in the market during the studied years. CDMOs and dedicated vector production facilities supply the high-quality viral vectors required for clinical and commercial use. They bridge manufacturing gaps for therapy developers, enabling rapid scale-up and regulatory compliance. Their importance continues to rise as global demand for AAV vectors outpaces in-house capabilities.
Recent Developments
- In June 2025, researchers from the University of California, San Diego, developed a novel AAV9-Synapsin-promoted Cav-1 (SynCav-1) to study their effects for Alzheimer’s disease (AD) in mice. They found that the therapy could be an effective way to protect the brain from disease-related damage and preserve cognitive function.
- In November 2024, the U.S. FDA approved the first AAV gene therapy delivered directly into the brain to treat AADC deficiency in children and adults. The therapy was developed by PTC Therapeutics. Kebilidi (eladocagene exuparvovec-tneq) is an AAV type 2-based gene therapy.
Browse More Insights of Towards Healthcare:
The global cell and gene therapy (CGT) pharmaceuticals market was valued at US$ 16.75 billion in 2024, expected to rise to US$ 19.91 billion in 2025, and projected to reach approximately US$ 91.56 billion by 2034, growing at a strong CAGR of 18.93% during the forecast period.
The cell and gene therapy manufacturing QC market stood at US$ 2.66 billion in 2024, increased to US$ 3.11 billion in 2025, and is anticipated to reach nearly US$ 12.35 billion by 2034, advancing at a CAGR of 16.89% from 2025 to 2034.
The cell and gene therapy bioassay services market was valued at US$ 5.05 billion in 2024, expanded to US$ 5.67 billion in 2025, and is projected to achieve around US$ 16 billion by 2034, registering a CAGR of 12.24% during the forecast timeline.
The viral vector-based cell & gene therapy CDMO market reached US$ 142.77 million in 2024, grew to US$ 162 million in 2025, and is forecast to climb to about US$ 497.7 million by 2034, marking a CAGR of 13.44% between 2025 and 2034.
The cell and gene therapy tools and reagents market accounted for US$ 10.04 billion in 2024, rose to US$ 11.12 billion in 2025, and is expected to hit roughly US$ 27.3 billion by 2034, progressing at a CAGR of 10.76% over the forecast period.
The cell and gene therapy thawing equipment market recorded US$ 0.96 billion in 2024, is set to grow to US$ 1.1 billion in 2025, and projected to reach nearly US$ 3.56 billion by 2034, with a CAGR of 14.24% through 2034.
The gene editing cell line generation service market is on a robust growth trajectory, anticipated to generate substantial revenue gains reaching hundreds of millions over the 2025–2034 period.
The gene knockdown stable cell line service market was valued at US$ 715 million in 2024, expected to grow to US$ 774 million in 2025, and projected to attain approximately US$ 1,557 million by 2034, expanding at a CAGR of 8.16% throughout the forecast period.
The gene overexpression cell line construction service market stood at US$ 788.63 million in 2024, increased to US$ 848.01 million in 2025, and is estimated to reach around US$ 1,629.93 million by 2034, growing at a CAGR of 7.53% from 2025 to 2034.
AAV Gene Therapy Market Key Players List

- Novartis (AveXis)
- Spark Therapeutics (Roche)
- BioMarin Pharmaceutica
- Sarepta Therapeutics
- uniQure
- Regenxbio
- Audentes Therapeutics (Astellas)
- Voyager Therapeutics
- MeiraGTx
- Asklepios BioPharmaceutical (AskBio - Bayer)
- Krystal Biotech
- Genethon
- BridgeBio Gene Therapy
- Passage Bio
- Solid Biosciences
- Freeline Therapeutics
- Capsida Biotherapeutic
- LogicBio Therapeutics
- Taysha Gene Therapies
- Iveric Bio (part of Astellas)
Download the Competitive Landscape market report @ https://www.towardshealthcare.com/price/5810
Segments Covered in The Report
By Therapeutic Area
- Neurological Disorders
- Spinal Muscular Atrophy (SMA)
- Parkinson’s Disease
- Rett Syndrome
- Hematologic Disorders
- Hemophilia A and B
- Ophthalmic Disorders
- Leber Congenital Amaurosis (LCA)
- Retinitis Pigmentosa
- Muscular Disorders
- Duchenne Muscular Dystrophy (DMD)
- Metabolic Disorders
- Rare Genetic Disorders
- Mucopolysaccharidosis (MPS)
- Batten Disease
By Vector Serotype
- AAV9 – Preferred for CNS and systemic delivery
- Engineered/Hybrid Capsids
- AAV2 – Widely used in ophthalmology
- AAV8 – Liver-directed therapies
- AAV5
By Route of Administration
- Intravenous (IV)
- Intrathecal (IT)
- Subretinal
- Intramuscular
- Intracerebral
By Application Stage
- Commercialized Therapies
- Zolgensma (SMA)
- Luxturna (LCA)
- Roctavian (Hemophilia A)
- Preclinical Therapies
- Clinical-Stage Therapies
- Phase I
- Phase II
- Phase III
By Manufacturing Type
- In-house Manufacturing
- Contract Development & Manufacturing Organizations (CDMOs)
By End User
- Pharmaceutical & Biotech Companies
- CDMOs/Vector Production Facilities
- Academic & Research Institutes
- Hospitals (as treatment centers)
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/5810
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

